Welcome to Celestine!
We have our first lab member! Celestine Wenardy has joined the lab as a research technician. Learn more about Celestine here .
2025.11.24
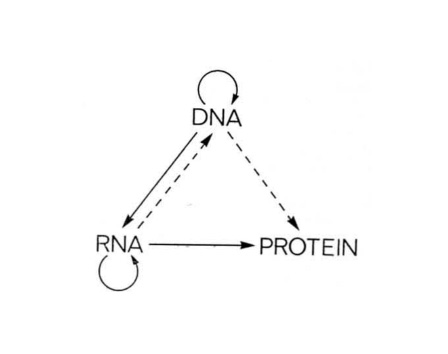
Genetic information typically flows in one direction: from DNA to RNA to protein. We are fascinated by reverse transcription, where this flow is flipped on its head and RNA is rewritten to DNA. Reverse transcriptases are famously used by retroviruses like HIV to invade genomes, but it turns out they are used by more than just genomic parasites.
We explore the frontiers of RNA biology by discovering new functions for reverse transcription. We already recognise that fundamental phenomena in molecular biology like splicing and telomere extension evolved from this ancient process, and we want to know: What else is out there? Using a combination of biochemistry, microbiology, and structural biology, we aim to discover new biological functions and harness them for applications in genomic medicine.
Just like us, bacteria are under constant threat of infection by viruses (called phages). We’ve found that many bacteria are armed with sophisticated viral defence systems powered by reverse transcription. While the mechanisms of these defences are largely mysterious, we recently discovered how one such system works. We found that upon phage infection, a bacterial reverse transcriptase synthesises a toxic repetitive gene from an RNA template. The single infected cell stops growing and thereby saves the entire bacterial population from the virus.
We are now investigating the mechanisms of other reverse transcriptase-based defence systems, to uncover what we expect to be a remarkable diversity of mechanisms that showcase the creativity of evolution.
Eukaryotic genomes, including our own, are littered with ‘jumping genes’ called retrotransposons. In fact, nearly 40% of the human genome is derived from a single type, the LINE-1 element. To understand these abundant elements, which are associated with genomic diseases and cancers, we study the widespread R2 element as a model system. R2 inserts itself specifically into ribosomal DNA arrays.
We have previously solved the structure of the R2 protein to reveal how it finds its target DNA and have shown that R2 can be used to direct therapeutic transgene insertion in human cells. We are now investigating the fundamental mechanisms of how R2 moves and how its expression is helped by and/or interferes with ribosome biogenesis. Our goal is to understand how these molecular parasites survive in a genome, and how we can adapt their activity for medicine.
We have our first lab member! Celestine Wenardy has joined the lab as a research technician. Learn more about Celestine here .
2025.11.24
The Wilkinson lab officially opened October 2025. The lab is part of the Structural Biology Program at Memorial Sloan Kettering Cancer Center, NYC.
2025.10.01